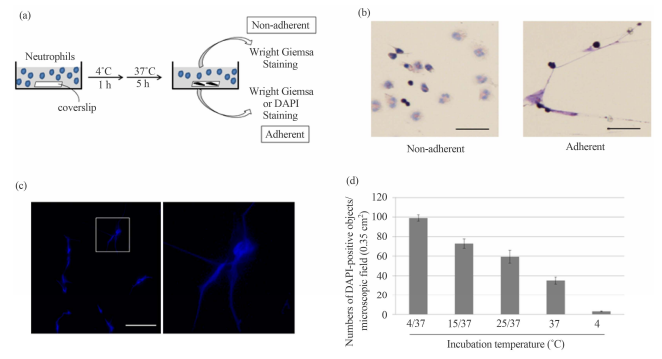
ABSTRACT By visualizing DNA with diamidinophenylindole (DAPI), we found that hypothermal incubation followed by rewarmingof human neutrophils resulted in an increased number of DAPI-positive objectsrepresentative of extensive DNA unfolding seemingly similar to neutrophilextracellular traps (NETs). In contrast to canonical NET formation, diphenyleneiodonium (DPI), an NADPH oxidase inhibitor, exhibited negligible effects onformation of the DAPI-positive objects. Moreover, multiple instances of DNAdamage were detected in the objects, but not in canonical NETs. Our results thussuggest the potential of hypothermia for triggering DNA structural alterationin neutrophils, which is similar to but distinct from NET formation. Keywords:Hypothermal Treatment; DNA Unfolding; Neutrophil Extracellular Trap (NET) 1.Introduction Low-temperature conditions, referred to as hypothermia, aregenerally used for the storage of cells, tissues, organs and bodies for bothscientific and clinical purposes. Hypothermia is an important means of slowingdown cellular metabolism during storage, thus inhibiting injurious processescaused by the deficiency of oxygen and substrate supply. However, hypothermiacan give rise to cell injury, including cell death [1,2]. Neutrophils are amain type of effector cell in the innate immune system [3,4], which circulatein the blood and engulf invading microorganisms such as bacteria and fungi byphagocytosis. In addition to such activities, Brinkmann et al. have reportedthat, following activation by microorganisms, neutrophils can undergomorphological changes detectable by microscopic observations [5]. These changesinclude loss of the lobular-shaped nucleus followed by disintegration of thenuclear envelope, which allow nuclear, cytoplasmic and granular components tomix together and subsequently rupture the cell membrane to release theDNA/chromatin into the extracellular environment [5]. The result is that theunfolded DNA/chromatin fibers with attached bactericidal proteins can functionas neutrophil extracellular traps (NETs) for microorganisms. NETs appear to bethe result of a unique form of cell death. Therefore, as opposed to apoptosisand necrosis, Steinberg and Grinstein coined the term “NETosis” for neutrophilcell death, which leads to the formation of NETs [6]. In addition tomicroorganism infection, several physiological inducers of NETs have beenreported [7 and references herein]. For instance, platelets activated viaTolllike receptor 4 rapidly induce NET formation [8]. Antibodies [9],antibody-antigen complexes [10,11], human immunodeficiency virus (HIV-1) [12],and microbial components such as lipopolysaccharide [13,14] are also known toinduce the formation of NETs. Although the intracellular signaling pathway(s)that transmit these physiological stimuli remain largely unknown, reactiveoxygen species (ROS) generation was demonstrated to be an absolute requirementfor NET formation [15,16]. Thus, one of the most widely-used agents to induceNETs in in vitro experiments is phorbol-12-myristate-13-acetate (PMA), whichdirectly stimulates protein kinase C (PKC) leading to potent activation ofnicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which in turngenerates superoxide [5,7,17]. Therefore, it is reasonable to use diphenyleneiodonium (DPI), a NADPH oxidase inhibitor [18], to block the formation ofPMA-stimulated NETs [15,17,19]. In this study, we found that hypothermalincubation of * Corresponding author. Copyright © 2013 SciRes. CellBio 118 J.KAWATA ET AL. human neutrophils at 4˚C for up to 1 h followed by incubation at37˚C resulted in an increased number of DAPI-stainable objects similar toglobal DNA unfolding observed in PMA-stimulated NETs. However, our additionalexperimental data revealed that hypothermia/rewarming-induced DNA unfolding wasregulated in a manner similar to, but biochemically and pharmacologicallydistinct from, canonical NETs. Although the molecular mechanism of thisphenomenon is not fully understood, we inferred, based on our experimentaldata, the possible role of ROS, which were generated during hypothermia/rewarming-treatmentin a manner independent of NADPH oxidase activity in the formation of theDAPI-positive, NET-like objects. 2. Materials and Methods 2.1. Peripheral BloodPreparation and Culture Human peripheral blood preparations (from two normalmale donors, collected in compliance with Kumamoto Health Science Universityand approved by the University Oversight Committee) were enriched forneutrophils by density gradient centrifugation with HISTOPPAQUE 117(Sigma-Aldrich) and Lymphocyte Separation Solution 1.119 (Nakarai Tesque)according to the procedures described by the supplier. Washed enrichedneutrophilic fractions were counted and examined for purity using Wright Giemsastaining (Sigma-Aldrich). 2.2. Drug and Hypothermal Treatments Cells were incubatedin culture dishes containing an immersed coverslip in RPMI 1640 (Sigma-Aldrich)supplemented with 5% fetal bovine serum (FBS), 1% penicillin/streptomycin and0.1% gentamaycin in a humidified atmosphere containing 5% CO2. To induce NETs,PMA (Wako Pure Chemical Industries) was added to the culture medium at aconcentration of 50 nM and incubated for 4 h at 37˚C. To inhibit NADPH oxidaseactivity, DPI (Cayman Chemical) was added to the culture medium at aconcentration of 20 μM. Hypothermal treatment and rewarming of cells wereperformed by incubation in a humidified atmosphere containing 5% CO2. Afterdrug and/or hypothermal/rewarming treatment, the coverslips were removed fromthe cultures and subjected to appropriate assays. DNA was visualized by stainingwith DAPI. 2.3. Antibodies and Immunostaining Cells were washed once for 5 minwith ice-cold PBS and then fixed with 4% paraformaldehyde in PBS for 5 min atroom temperature. After fixation, the cells were rinsed once with PBS andsubjected to indirect-immunofluorescence analysis using anti-neutrophilelastase (Calbiochem), anti-histone H3 (Santa Cruz Biotechnology), andanti-histone H3 citrulline R26 (Abcam) antibodies. The secondary antibodieswere obtained from Santa Cruz Biotechnology and Sigma-Aldrich. 2.4. BacteriaTrapping Assay Escherichia coli BL21 (DE3) were transformed with pET28-EGFP, aplasmid for expression of green fluorescent protein (GFP), and cultured inLuria-Bertani (LB) medium containing kanamycin at 37˚C for 16 h. 107 E. coli cellswere incubated with a coverslip containing hypothermia/rewarming-inducedDAPI-positive objects in RPMI 1640 supplemented with 5% FBS at 37˚C. After 20min at room temperature, the coverslips were washed three times with PBSfollowed by incubation with 4% paraformaldehyde. DNA fibers were stained withDAPI. Because the E. coli expressed GFP, bacteria trapped by DNA fibers couldbe detected by fluorescence microscopy. For DNase I treatment, the coverslipswere treated with PBS containing 100 U/ml DNase I (Takara) at 37o C for 1 hr.The numbers of E. coli with GFP signals on the coverslips were counted byfluorescent microscopy. 2.5. TUNEL Assay Terminal deoxynucleotidyl transferasedUTP nick end labeling (TUNEL) assays were performed using the MEBSTAIN ApoptosisTUNEL Kit II (MBL) according to the manufacturer’s instructions. TheTUNEL-positive cells were counted under a microscope. The percentage ofTUNEL-positive cells was defined by the number of positive cells among thetotal number of cells in each sample. For one experiment, cells were counted inat least three different microscopic fields of view. 2.6. Intracellular ROSDetection Assay Intracellular ROS production was monitored using the cellpermeable fluorescent dye, CellROX Deep Red Reagent (Invitrogen). This agentcan readily react with ROS to form a fluorescent product proportional to theamount of ROS generated in the cells. The cells were incubated with 5 μMCellROX Deep Red for 30 min and then harvested. The fluorescence intensity ofthe cells was measured using a FACSVerse flow cytometer (BD Biosciences). 2.7.MitoTracker Analysis After fixation with 4% paraformaldehyde, cultured humanneutrophils were stained with the mitochondrionspecific dye, MitoTracker RedCMXRRos (Invitrogen), according to the manufacturer’s instruction. The cellswere immediately analyzed using a FACSVerse flow cytometer. Copyright © 2013SciRes. CellBio J. KAWATA ET AL. Copyright © 2013 SciRes. CellBio 119 2.8.Statistical Analysis Wright–Giemsa staining (Figure 1(b), left panel). Incontrast, we unexpectedly found that small, but substantial, numbers ofWright-Giemsa-stainable materials, which looked different from intactneutrophils, were present on the coverslip (Figure 1(b), right panel). Unlessotherwise stated, all data are presented as the mean ± SD. Within inpidualexperiments, data points were based on a minimum of triplicate representativesamples and experiments were repeated at least three times. When the materialson the coverslip were stained with DAPI without any fixative treatment, weobserved bright fluorescent signals under fluorescent microscopy, many of whichappeared to consist of multiple DAPI-positive strings (Figure 1(c)). BecauseDAPI is a fluorescent dye that intercalates into double-stranded DNA, and thatliving neutrophils are less permeable to the dye than dead neutrophils, wethought it probable that these bright DAPI-stained signals represented globalDNA unfolding of dead neutrophils, which somehow adhered to the coverslip. Itshould be mentioned that there were few neutrophils with normal morphology onthe coverslip per view field, implying that most of neutrophils floated in theculture medium under the standard culture conditions. 3. Results and Discussion3.1. Effect of Hypothermia on Human Neutrophils in Culture After isolatinghuman neutrophils from peripheral blood preparations (see Materials andMethods), the cells (4.5 × 106 cells) were incubated at 4˚C for 1 h followed byincubation at 37˚C for 5 h in the 6-cm culture dish supplemented with 2 ml ofthe culture medium, in which a coverslip was immersed (Figure 1(a)). We foundthat most of the cells were present as non-adherent forms, and were thusfloating in the culture medium. These non-adherent cells appearedmorphologically intact as shown by Figure 1. DAPI-stained objects in thehypothermia/rewarming-treated human neutrophils. (a) Schematic representationof procedure for detecting the hypothermia/rewarming-induced DAPI-positiveobjects. Human neutrophils from peripheral blood preparations were cultured indishes containing a coverslip at 4˚C for 1 h followed by rewarming at 37˚C for5 h. Non-adherent and adherent materials in the culture medium stained withWright Giemsa; (b) Non-adherent and adherent materials in the culture were stainedwith Wright Giemsa (left and right panels). Bar indicates 50 μm; (c) Themorphologies of the DAPI-stained objects adherent to the coverslip weredetected by fluorescence microscopy (left panel). Bar indicates 50 μm. Thepanel on the right shows a higher magnification of the region indicated in theleft panel; (d) Human neutrophils from peripheral blood preparations (1 × 106cells) were cultured in dishes containing a coverslip for 6 h at 4˚C (4), at4˚C for 1 h followed by rewarming at 37˚C for 5 h (4/37), at 15°C for 1 hfollowed by rewarming at 37˚C for 5 h (15/37), at 25˚C for 1 h followed byrewarming at 37˚C for 5 h (25/37), and at 37˚C for 6 h (37). After incubationunder the conditions as indicated, the numbers of DAPI-positive objects on the coverslipsin the microscopic field (0.35 cm2 ) were counted. The values shown representmeans ± SE of three independent experiments. 120 J. KAWATA ET AL. We theninvestigated whether the requirement for DAPI-positive object production wassimple exposure to hypothermia or rather the combination of hypothermia/rewarming. When human neutrophils were maintained at a constant temperature ofeither 4˚C or 37˚C, significantly less DAPI-positive signals were detected ascompared with cells cultured either at 4˚C, 15˚C, or 25˚C for 1 h followed byincubation at 37˚C for 5 h (Figure 1(d)). These results suggest that theappearance of the DAPIpositive objects was associated with incubation ofneutrophils under hypothermal conditions followed by rewarming. 3.2. Comparisonof the Biochemical and Immunohistochemical Properties ofHypothermia/Rewarming-Induced DAPI-Positive Objects and PMA-Stimulated NETsWhen we observed the DAPI-positive objects in hypothermia/rewarming-treatedhuman neutrophils, we noticed that morphological similarities between theobjects and DAPI-stained PMA-stimulated NETs, leading us to suspect that theDAPI-positive objects per se might represent NETs (Figure 2(a)). To investigatethis possibility, we first asked whether the that DAPI-positive objectspossessed the ability to bind bacteria. Given NETs are defined as extracellularDNA-proteinaceous structures that exhibit the ability to associate with a widevariety of Gram-positive and Gram-negative pathogens [7], we expected that theDAPI-positive structures might also show similar properties. As shown in Figure2(b), when GFP-expressing E. coli was incubated with the coverslip containingDAPI-positive objects, we found multiple GFP signals present together with theDAPI-signals. Their ability to trap bacteria appeared equivalent to that ofPMA-stimulated NETs, because the number and distribution of GFP-signalsassociated with the DAPI-positive objects were very similar to those associatedwith PMA-stimulated NETs, suggesting that the objects possessed the ability totrap bacteria. It should be mentioned that the number of bacteria trapped tothe DAPI-positive objects was reduced when the coverslips were treated withDNase I (Figure 2(c)). Similar results were obtained when PMA-stimulated NETswere treated with DNase I. These results imply that both structures are equallysusceptible to DNase I treatment with respect to bacterial trap. To furtherevaluate the similarities between the DAPIpositive objects and canonical NETs,we performed indirect-immunofluorescence analysis using antibodies thatrecognize marker proteins for NETs: anti-neutrophil Figure 2.Hypothermia/rewarming-induced DAPI-positive objects exhibited several featuressimilar to PMA-stimulated NETs. (a) Human neutrophils were incubated in aculture dish containing a coverslip at 4˚C for 1 h followed by incubation at37˚C for 5 h. The coverslip was removed and fixed in PBS containing 4%paraformaldehyde and then stained with DAPI (left). For the control,PMA-stimulated neutrophils, which exhibit canonical NETs, were fixed with 4%paraformaldehyde and subjected to DAPI-staining (right). Bar indicates 50 μm;(b) Human neutrophils were incubated in a culture dish containing a coverslipat 4˚C for 1 h followed by incubation at 37˚C for 5 h. The coverslip wasremoved and then transferred to culture medium containing E. coli expressingrecombinant GFP, followed by incubation for 15 min at 37˚C (left). For thecontrol, PMA-stimulated neutrophils were treated in the same way (right). Thearrows indicate GFP-signals that represent E. coli. Bar indicates 50 μm; (c)After hypothermia/rewarming-(left) or PMA-incubation (right), the coverslipswere treated with DNase buffer alone (gray bars) or DNase buffer containing 100U/ml DNase I (black bars) at 37˚C for 1 hr. The numbers of E. coli with GFPsignals on the coverslips in the microscopic field (0.35 cm2 ) were counted.The values shown represent means ± SE of three independent experiments; (d) Thehypothermia/rewarming-induced DAPI-positive objects and PMAstimulated NETs wereimmunostained with (upper panels in left and middle-right columns) or without(upper panels in middle-right and right columns) anti-NE antibodies.DAPI-stained images of each treatment are shown at the bottom. Bar indicates 50μm; (e) The hypothermia/rewarming-induced DAPI-positive objects andPMA-stimulated NETs were immunostained with (upper panels in left andmiddle-right columns) or without (upper panels in middle-right and rightcolumns) anti-histone H3 antibodies. DAPI-stained images of each treatment areshown at the bottom. Bar indicates 50 μm. Copyright © 2013 SciRes. CellBio J.KAWATA ET AL. 121 elastase (NE) and anti-histone H3 antibodies [7]. As shown inFigures 2(d) and (e), in the presence of these anti-bodies, the signals weredetected not only on PMAstimulated NETs but also on the DAPI-positive objects.In contrast, in the absence of the antibodies, these signals were barelydetected, suggesting existence of NE and histone H3 on both the DAPI-positiveobjects and canonical NETs. Taken together, at least with regard to theirability to trap bacteria and the existence of two marker proteins for NETs, ourresults indicated that the DAPI-positive objects possessed biochemicalsimilarities to canonical NETs. It should be noted, however, the experimentsdescribed above are not sufficient to conclude that the DAPI-positive objectshave anti-bacterial activity. We are therefore investigating whether NE andhistones on the DAPIpositive objects can indeed inactivate bacterial toxic proteins,called “virulence factors,” and inhibit bacterial growth. In addition, we wishto test whether the DAPIpositive objects can capture microorganisms besidesGram-negative bacteria (E. coli), such as fungi and parasites. 3.3. TheDAPI-Positive Objects Exhibited Several Biochemical Properties Different fromThose of PMA-Stimulated NETs Although our results so far indicated acorrelation between DAPI-positive objects and NETs, several differences werealso revealed. For instance, when indirect immunofluorescence analysis wasconducted using antihistone H3 citrulline R26 (anti-H3cit) antibody, we foundthat the antibody stained many, but not all, PMAstimulated NETs, whereas theantibody proved poor at detecting the DAPI-positive objects (Figure 3(a)). Becauseit has been reported that peptidylarginine deiminase 4 (PAD4/PADI4), whichcatalyzes histone hy percitrullination, mediates chromatin decondensation andis vital to NET formation [13,20,21], our observation of different stainingpatterns with anti-H3cit antibody between the DAPI-positive objects andPMA-stimulated NETs implies activation of PAD4 in PMA-stimulated cells, whilethe enzyme might not be fully activated in the hypothermia/rewarming-treatedcells. We also detected differences between the objects and NETs in TUNELassays. As shown in Figure 3(b), TUNEL-positive signals were negligible inPMA-treated cells, confirming the previous report that no TUNELpositive DNAdamage is detectable in canonical NETs [15]. In contrast, TUNEL assay visualizedmore than 90% of the hypothermia/rewarming-induced DAPI-positive objects,indicating the existence of multiple sites of TUNEL-positive DNA damage onextensively unfolded DNAs in the objects. Because TUNEL-positive signals arefrequently associated with apoptotic cells, these data indicated thathypothermia/rewarming-incubation might trigger, to some extent, activation ofapoptosis-related DNA cleavage enzyme(s) in neutrophils, suggesting thepossible contribution of apoptotic signaling pathways, at least in part, to DNAstructural alterations during the formation of the DAPI-positive, NET-likestructures. 3.4. Involvement of ROS Elevation to Generate the DAPI-PositiveObjects Given that ROS generation is an absolute requirement for the formationof NETs [15,16], we next assessed whether hypothermia/rewarming of neutrophilscoincided with the generation of ROS. Thus, we measured ROS inhypothermia/rewarming-treated human neutrophils. As shown in Figure 4(a),during constant temperature incubation at either 4˚C or 37˚C, noincrease/decrease in ROS was detected. In contrast, we found a significantelevation of ROS when cells were kept at 4˚C followed (a) (b) Figure 3.Hypothermia/rewarming-induced DAPI-positive, NET-like structures exhibitedseveral features distinct from PMA-stimulated NETs. (a) Thehypothermia/rewarming-induced DAPI-positive objects and PMA-stimulated NETswere immunostained with (upper panels in left and middle-right columns) orwithout (upper panels in middle-right and right columns) anti-H3cit antibodies.DAPI-stained images of each treatment are shown at the bottom. Bar indicates 50μm; (b) Hypothermia/rewarming-induced DAPI-positive, NET-like structures (leftcolumn) and PMA-stimulated NETs (right column) were subjected to TUNEL assay(upper panel). DNA was visualized with propidium iodide (PI; lower panel). Barindicates 50 μm. Copyright © 2013 SciRes. CellBio 122 J. KAWATA ET AL. (a) (b)(c) Figure 4. Hypothermia/rewarming-induced DAPI-positive, NET-like structuresare biochemically and pharmacologically distinct from PMA-stimulated NETs. (a)Human neutrophils were incubated at 4˚C for 1 h followed by incubation at 37˚Cfor 1 h (indicated as “1/1”) or 3 h (indicated as “1/3”) in culture medium with(+; black bars) or without (−; gray bars) 20 μM DPI. ROS formation by cellsincubated at 4˚C and 37˚C was quantified using CellROX Deep Red Reagent andfold ROS generation (ROS formation at 37˚C over that at 4˚C) was calculated.For the control, ROS formation was quantified during cell culture at 4˚C for 1h (indicated as “1/0”) and 37˚C for 1 h or 4 h (indicated as “0/1” or “0/4”,respectively) in the presence or absence of DPI, and fold ROS generation duringeach culture period (ROS formation at the start over that at the end ofculture) was calculated; (b) Human neutrophils (1 × 106 cells) were incubatedat 4˚C for 1 h followed by incubation at 37˚C for 5 h in culture medium with(black bars) or without (gray bars) 20 μM DPI. The numbers of DAPI-positiveobjects on the coverslips in the microscopic field (0.35 cm2 ), which were inproportion to total numbers of the DAPI-positive objects in the cultures, werecounted. For the control, human neutrophils were incubated at 37˚C in thepresence (+) or absence (−) of 20 μM DPI or 50 nM PMA and the numbers of NETswere counted; (c) Human neutrophils were incubated at 4˚C for 1 h followed byincubation at 37˚C for 1 h (indicated as “1/1”) or 3 h (indicated as “1/3”) inculture medium with (+; black bars) or without (−; gray bars) 20 μM DPI. Cellswere subjected to MitoTracker Red-staining and fold changes in fluorescentsignals (signal at 37˚C over that at 4˚C) were calculated. For the control,fluorescent signals were quantified during cell culture at 4˚C for 1 h(indicated as “1/0”) and 37˚C for 1 h or 4 h (indicated as “0/1” or “0/4”,respectively) in the presence or absence of DPI, and fold fluorescent signalchanges during each culture period (signal at the start over that at the end ofculture) were calculated. by incubation at 37˚C for 1 h or 3 h; an approximate10- fold increase in ROS was apparent in the cells after incubation at 37˚C. Wenext examined whether NADPH oxidase contributed to ROS production inhypothermia/rewarmingtreated cells. Given that PMA-induced NET formation iseffectively inhibited by DPI, an inhibitor of NADPH oxidase activity [15,17,18and see Figure 4(b)], we tested the effect of this drug. Intriguingly, neitherROS generation nor the formation of DAPI-positive objects was significantlyaffected by administration of DPI (Figures 4(a) and (b)), indicating that themechanism of ROS generation in hypothermia/rewarming-treated neutrophils couldbe pharmacologically distinguished from that in PMA-stimulated cells withrespect to the involvement of NADPH oxidase in ROS generation. Copyright © 2013SciRes. CellBio J. KAWATA ET AL. 123 Although where and how ROS are produced inthe hypothermia/rewarming-treated cells remains unclear, it is noteworthy thatthe mitochondrion-specific dye, MitoTracker Red, detected structural and/orfunctional alterations in mitochondria in the hypothermia/rewarmingtreatedcells (Figure 4(c)). Because mitochondria are known to produce ROS in aerobicorganisms, including humans [22], these data suggest scenario that thisorganelle may generate ROS during hypothermia/rewarming, leading to theformation of the DAPI-positive, NET-like structures. However, DPI is also knownas a potent inhibitor of mitochondrial reactive oxygen species production [23].If DPI inhibits generation of ROS from both mitochondria and the NADPH oxidasepathway during hypothermia/rewarming of neutrophils, we need to consider thepossibility of an alternative pathway to generate ROS, besides the NADPHoxidase pathway or the mitochondria pathway. 4. Conclusion DAPI-positiveobjects with extensive DNA unfolding were observed in human neutrophilscultured in hypothermic conditions followed by rewarming. Our experimental dataindicated that such DNA structural alterations in neutrophils may be related toNET formation, but can be biochemically and pharmacologically discriminatedfrom NET formation. We also considered that the objects might not representapoptotic cells, given that apoptotic cells contain condensed DNA enclosed inmembrane, which is not observed in the objects. We thus suggest that thehypothermia/rewarming-induced DNA unfolding is regulated in a manner distinctfrom either canonical NETosis or canonical apoptosis, arguing the existence ofa previously unappreciated signaling pathway that alters global genomic DNAstructures in eukaryotic cells. Further, the results indicate thatcoldtreatment followed by warming may affect NET formation, which is animportant consideration because many researchers use hypothermal conditionsduring the isolation and culture of neutrophils. 5. Acknowledgements We thankall the members of the Saitoh Laboratory for helpful discussion. This work wassupported by research grant to H. S. from Astellas Foundation for Research onMetabolic Disorders, and by intramural founding in Kumamoto Health Science Universityto J. K.